ACRODERMATITIS ENTEROPATHICA LIKE: CASE REPORT
Abstract.
The present study is a case report of Acrodermatitis Enteropathica-like syndrome of a patient who is female, white, and was detected at 5 months of age and treated with zinc supplementation. The etiology may be due to an inborn error of metabolism configured by autosomal recessive inheritance, or due to personal and/or environmental factors. The monitoring of the infant until total suspension of therapy is necessary to verify episode or non-recurrence of the condition.
Keywords
Acrodermatitis; Zinc Deficiency; Metal Metabolism, Inborn Errors
Introduction
Acrodermatitis enteropathica is a disease resulting from deficiency of zinc absorption from the gastrointestinal tract, causing low serum values of this element. Its etiology may be due to an inborn error of metabolism configured by autosomal recessive inheritance, or due to personal and/or environmental factors such as prematurity, malabsorption syndrome, short bowel, low zinc intake, and other factors.
Zinc is an essential element in several metabolic functions and the lack of it triggers acral and periorificial skin lesions, chronic diarrhea, weight loss, alopecia, growth retardation and immunodeficiency
This paper reports a case of Acrodermatitis Enteropathica at the Hospital of Pemphigus in the city of Uberaba, Minas Gerais, Brazil, in 2013. It is justified by the fact that this disease has a low incidence in the population, often observed in specific groups, such as premature babies hospitalized in intensive care units and the elderly.
The following databases have been used: Medline, SciELO, Lilacs-Bireme and Cochrane and the works have been selected according to their relevance from 1974 to 2015. The parents of the patient have also signed the informed consent form (ICS).
Case Report
AAC, female, caucasian, 5 months old, born in São José do Rio Preto, state of São Paulo (SP), originally Fernandópoles/SP. Born of caesarean section at 31 weeks due to maternal placental insufficiency, with birth weight of 1.300 kg, hospitalized in the intensive care unit for 55 days from birth. Exclusive breastfeeding.
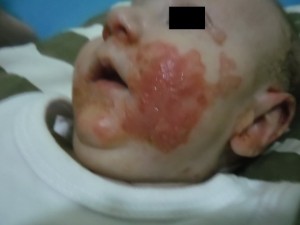
In June 2013 the infant started to show reddish spots in the perilabial region with subsequent evolution to similar periungual stain on the hands and feet and finally reaching genital and lower limb regions. The stains gradually increased in size, as shown in Picture 1.
Source: author.
The infant stool had a soft appearance and and difficulty to gain weight. She had no other systemic symptoms. The situation did not improve with topical or oral antibiotics and antifungals or with corticosteroids and topical immunomodulators.
Physical examination was presented in good general condition, ruddy, hydrated, anicteric, acyanotic, emaciated. Distended abdomen, tympanic, normal bowel sounds, absence of visceromegaly. Cardiorespiratory system had no alterations. Reddish stains have been observed, of well-defined edges, bright, humid in perilabial regions, left ocular edge, periauricular, ears, buttocks, groin and labia majora, right and left thighs, phalanges, periungual regions of hands and feet, arms and legs and posterior occipital region. The infant had also a pressure ulcer in the lower back.
The laboratory test presented serum level of zinc of 63.4 (70 to 120 mg/dL) as of September 4 2013.
Zinc replacement started on September 7 2013, in the form of gluconate, at a dose of 100 mg per day orally. Eight days after the start of zinc replacement there had been significant lightening of stains and remission of some injuries, in addition to improvement in the general state of the infant.
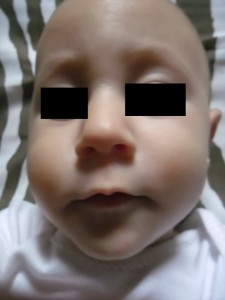
Lastly, twenty-two days after the start of the treatment, it was possible to observe almost 100% remission of the stains, as shown in Picture 2.
Source: author.
It must be emphasized that as the clinical aspect was improving, her serum levels of zinc were also increasing, reaching the end of treatment with physiological levels of serum zinc (serum zinc value (mg/dL), average: 70-120 mg/dL), according to zinc dosage method: Atomic Absorption Spectroscopy.
Serum zinc levels since start of medical treatment: September 4 2013 – 63.4 mg/dL, October 7 2013 – 121.3 mg/dL, November 7 2013 – 92.6 mg/dL, December 11 2013 – 128.4 mg/dL, January 13 2014 – 105.1 mg/dL, March 10 2014 – 156.0 mg/dL, April 22 2014 – 174.5 mg/dL, June 12 2014 – 213.0 mg/dL, August 10 2014 – 216.0 mg/dL, October 22 2014 – 122.1 mg/dL, December 10 2014 – 85.7 mg/dL.
On April 29 2014 the zinc gluconate dose had been reduced to 80 mg per day. In June 21 2014 the zinc gluconate dose had again been to 60 mg per day. On August 25 2014 the zinc gluconate dose had been reduced to 40 mg per day. On October 29 2014 the dose was further reduced to 30 mg per day and so it remained until January 2015.
The patient had no recurrence of the injuries and evolved with weight gain and significant improvement in general health and neuropsychomotor development.
Discussion
Zinc is one of the main chemical elements in humans that exert catalytic, structural and regulatory functions.¹ It is an essential cofactor for many enzymes throughout metabolism and plays an important role in growth and development, in cellular proliferation and tissue repair. It also has an important role in the immune system by depressing the cytotoxic activity of lymphocyte T killers and the phagocytic and bactericidal capacity of neutrophils.2-9
The term Acrodermatitis Enteropathica (AE) refers to a rare disease of autosomal recessive origin, where a disorder in zinc absorption occurs, typically observed after weaning, requiring zinc replacement throughout life.10-16 World distribution has an estimated incidence of 1 in 500,000 live births, and there is no predilection for gender nor race. Exclusive breastfeeding is a protective factor and an effective therapy due to the presence of a low molecular weight binding, which increases zinc absorption in the gastrointestinal tract of infants.2,10,11,16-18 At first, the mutation of a gene has been reported in mice and named “lethal milk” (lm).2,10,11,19,20 After that, the acrodermatitis enteropathica gene was isolated, named SLC39A4, located in 8q24-3 chromosome region, which encodes the zinc transporter, Zip 4.5,12,13,16 19
In contrast, the transient and symptomatic zinc deficiency (AE like), self-limited situation, usually occurs between the 8th and 24th week of life and can occur even in children on exclusive breastfeeding, premature or full term. In most cases, it is due to low amounts of zinc in breast milk, despite maternal serum levels being normal.1,10,11,14,15,18 Furthermore, after zinc supplementation, there had been an increase in serum level but not in breast milk. A defective secretion of zinc is observed by the mammary glands due to ineffective absorption of plasma zinc to the breast, then suggesting a deficiency or malfunction of the zinc binding.1,10,12,15,20 Studies indicate that the zinc transporter gene SLC30A2 (ZnT2) is responsible for this defective transfer.19
The zinc concentration in breast milk typically falls exponentially during the course of lactation. The concentration in the first week is 80 to 110 mmol/L and in the fourth, 30 to 80 mmol/L. The minimum acceptable level of the concentration in the 20th week is 11 to 12 mmol/L. The concentration of zinc in breast milk of mothers of premature or full term infants is not different.11,12
There are cases described in the literature of premature in exclusive breastfeeding that develop zinc deficiency symptoms, and whose breast milk has normal levels of zinc.4,10,11 These cases are more complex due to numerous physiological and organic factors. The large zinc accumulation occurs in the 3rd trimester of pregnancy, therefore, the amount of body zinc is inversely proportional to the degree of prematurity. In addition, premature infants are prone to a negative zinc balance until 60 days of life, secondary to poor absorption of zinc, increased intestinal secretion of zinc, increased zinc demand due to the fast growth and development and high loss of this element in feces.1,2,4,10,12,18 This situation was more observed in premature infants between 25 and 33 weeks of gestation.11
The transient symptomatic zinc deficiency and Acrodermatitis Enteropathica share the same clinical characteristics, and include erythematous rash, vesiculobullous, or psoriasiform, symmetrical, in perioral and perineal regions, with acral distribution of lesions, paronychia, onychodystrophy, soft stools, alopecia, fever, growth disorders, conjunctivitis and behavioral changes such as irritability and prostration.2-4,6-11,13,15-17
In transient symptomatic zinc deficiency, the effect of zinc supplementation is rapid, taking only 3 days to 2 weeks to show lightening of the skin injuries. Treatment in these cases is kept until weaning. In contrast, in congenital Acrodermatitis Enteropathica zinc supplementation is for life.2,11
However, it is still important to report the differences between the variants of congenital acrodermatitis enteropathica and acquired acrodermatitis enteropathica, that until nowadays, are considered diseases of diagnostic importance. It is also important to recognize the etiology of zinc deficiency in infants.11
Congenital: Acquired
1) Acrodermatitis Enteropathica: Inadequate supplementation of zinc:
- a) Low level of zinc in breast milk;
- b) Total parenteral nutrition with low level of zinc.
2) Prematurity:
- a) Low accumulated amount of zinc (obtained after 3rd trimester);
- b) Low absorption of zinc and high fecal loss.
3) Bad absorption:
- a) Cystic fibrosis;
- b) HIV infection.
Besides the differences presented, it is important to highlight other causes of zinc acquired deficiency which can result in skin injuries similar to those previously reported: Syndromes of intestinal malabsorption, extensive burns, Crohn’s disease, sickle cell anemia, celiac disease, systemic malignancy, pancreatic insufficiency, renal tubular dysfunction, drugs, defects in the mammary secretion of zinc, short bowel syndrome, diets rich in phytates (leguminous) and calcium, total parenteral nutrition.3
The AE has as differential diagnosis: psoriasis, seborrheic dermatitis, atopic dermatitis, contact dermatitis, impetigo, mucocutaneous candidiasis, histiocytosis X, biotin deficiency and of multiple carboxylases.2,6,11
The treatment of choice is zinc supplementation, which can be done through oral preparations based on acetate, gluconate, sulfate or amino acid chelate, in an average dose of 1 to 2 mg of elemental zinc/kg/day.2,12,17
The case described in this paper is probably a transient and symptomatic zinc deficiency due to prematurity, although it is not possible to establish the precise cause due to unknown zinc dosage in breast milk. It was possible to reach this conclusion because of the following factors:
– Beginning of skin injuries with 3 months of life, when the infant was still in exclusive breastfeeding;
– Prematurity (31 weeks) and long period of stay in Intensive Care Unit;
– Quick improvement with zinc supplementation;
However, it is still necessary to monitor the infant until full suspension of therapy, to verify if there is recurrence of the situation, that if it occurs, speaks in favor of Acrodermatitis Enteropathica. Not less important is to track future pregnancies of the mother, due to a possible deficiency in zinc binding in the mammary glands, which, if any, will cause the same clinical condition in subsequent pregnancies.
References
- Stevens J, Lubitz L. Symptomatic zinc deficiency in breast-fed term and premature infants. J Paediatr Child Health. 1998; 34: 97-100.
- Kiechl-Kohlendorfer U, Martin Fink F, Steichen-Gersdorf E. Transient symptomatic zinc deficiency in a breast-fed preterm infant. Paediatr Dermatol. 2007; 24(5): 536-40.
- Perafán-Riveros C, Sayago LF, Alves ACF, Sanches, JA. Acrodermatitis Enteropathica: case report and review of the literature. Pediatr Dermatol. 2002; 19(5): 426 – 31.
- Barbarot, S. Chantier E, Kuster A, Hello M, Roze JC, Blouin E et al. Symptomatic acquired zinc deficiency in at-risk premature infants: high dose preventive supplementation is necessary. Pediatr Dermatol. 2010; 27(4): 380 – 3.
- Sanchez JE, Barham KL, Sangueza OP. Acquired acrodermatitis enteropathica: case report of an atypical presentation. J Cut Pathol. 2006; 34: 490 – 3.
- Mostafa WZ, Al-Zayer AA. Acrodermatitis enteropathica in Saudi Arabia. Int J Dermatol. 1990; 29(2):134 – 8.
- Lee SY, Jung YJ, Oh TH, Choi EH. A case of acrodermatitis enteropathica localized on the hands and feet with a normal serum zinc level. Ann Dermatol. 2011;23: S88-90.
- Prasad AS. Zinc in human health: effect of zinc in immune cells. Mol Med. 14: 353 – 7.
- Brocard A, Dreno B. Innate immunity: a crucial target for zinc in the treatment of inflammatory dermatosis. J Europ Acadof Dermatol and Venereol. 2011; 25:1146 – 52.
- Young HS, Khan ASA, Power S, Ehrhardt P, Coulson IH. Case 4. Clin Exper Dermatol. 2003; 28: 109-10.
- Stapleton KM, Toughlin OE, Relic PJ.Transient zinc deficiency in a breast-fed premature infant. Austral J Dermatol.1995; 36: 157 – 59.
- Azevedo P M C, Gavazzoni-Dias MFR, Regazzi Avelleira JCR, Lerer C, Sousa AS, Azulay DRAzevedo P M C. Acrodermatitis enteropathica in a full-term breast-fed infant: case report and literature review. Internat J Dermatol. 2008; 47: 1056-7.
- Chue C D, Rajpar S F, Bhat J. Na acrodermatitis enterophatica-like eruption secondary to acquired zinc deficiency in an exclusively breast-fed premature infant. Internatl J Dermatol. 2008; 47:372-3.
- Tatlican S, Yamangokturk B, Eren C, Gulbahar O, Fatma Eskioglu F. A diagnostic challenge: a case of acrodermatitis enteropathica without hypozincemia and with maternal milk of low zinc level. Pediatr Dermatol. 2010; 27 (5): 534 – 5.
- Agarwal S, Gopal K. Acrodermatitis enteropathica in a breast-fed infant. Depart Dermatol Venereol. 2007; 77: 209.
- Karadag A S, Bilgili S G, Calka O. Acrodermatitis enteropathica in three siblings. Indian J Dermatol Venereol. 2013; 79: 268 – 9.
- Kharfi M, El Fékih N, Aounallah-Skhiri H, Schmitt S, Fazaa B, Kury S, Kamoun MR. Acrodermatitis enteropathica: a review of 29 Tunisian cases. Int J Dermatol. 2010; 49: 1038 – 44.
- Chew AL, Chan I, McGrath JA, Atherton DJ. Infantile acquired zinc deficiency resembling acrodermatatis enteropathica. Clin Experiment Dermatol. 2005; 30: 578 – 602.
- Santiago F, MatosJ, Moreno A, Schmitt S, Bezieau S, Tellechea O. Acrodermatitis enteropathica: a novel SLC39A4 gene mutation found in a patient with an early-onset. Pediatr Dermatol. 2011; 28 (6): 735 – 36.
- Radja N, Charles-Holmes R. Acrodermatitis enteropathica – lifelong follow-up and zinc monitoring. Clin Experiment Dermatol. 2002; 27: 62 – 3.
Attachments
Picture 1: Initial stage of the lesion development in September 2013. Erythematous rash, bright and well defined edges, periorificial.
Picture 2: May 2014. Six months after the initial stage of zinc supplementation. Total lightening of injuries, without sequelae.
Authors
Kelly Dematte Silva Mattede1; Lusvarghi B H M Beatriz Helena Martins Lusvarghi2; Bárbara Regina Martins Lusvarghi3*
1 Post graduate in Dermatology – (Medical Doctor).
2 Post graduate in Dermatology – (Doctor at Poços de Calda’s City Council. Minas Gerais).
3 Pediatric specialist – (Pediatric Doctor at Mário Palmério Universitary Hospital of Federal University of Uberaba. Minas Gerais).