ACUTE AND CHRONIC EFFECTS OF LEAD ON CONDUCTANCE VESSELS
Abstract
This study aims to demonstrate the main effects of lead exposure on the vascular system emphasizing actions on conductance vessels. For this purpose, a narrative review was carried out with two main axes: 1) guiding question: which are the effects of acute and chronic exposure to lead on conductance vessels in humans and experimental animals: 2) review of the literature and selection of relevant studies related to this subject. The studies presented in this review showed that lead, regardless of the time of exposure, promotes vascular alterations that are directly associated with an increase in blood pressure contributing to the development of arterial hypertension. Another important aspect is that in all the studies that were performed in the blood analysis after exposure to lead, values well below those recommended as safe by the toxicovigilance agency of Brazil and other countries were found. Research carried out in humans and experimental animals shows lead as an important risk factor for the development of cardiovascular diseases, and it is necessary to reduce the levels of exposure, recommended as safe, for the general population.
Introduction
Lead presents a high toxicity capable of promoting adverse effects on the human body. This metal is stored in the bones where it causes changes1 and can be stored in soft tissues such as: kidneys; adrenal glands; pancreas; gallbladder; ovaries; prostate; testicles, heart, blood vessels and skeletal muscle. The concentrations of this cation in these tissues appear to be constant throughout life, due to their high turnover rate.2 The toxic effects of lead are potentiated by factors such as age, nutritional factors due to iron deficiency and malnutrition, and the presence of concomitant diseases.3
Exposure to lead is widely recognized as a common occupational and environmental health problem.4 Clinical and epidemiological studies show a correlation between blood lead concentration and blood pressure. This is true even at low concentrations, such as 10 to 25 μg / dL Pb-S,5 similar to the values observed in a population exposed to this metal environmentally.6-8 The harmful effects of this metal on the cardiovascular system, with the development of diseases, compromise this system, producing changes in blood pressure8,9 coronary artery disease, myocardial infarction and peripheral arterial disease.9,10
According to the World Health Organization (WHO), cardiovascular diseases are currently the leading cause of mortality and morbidity in the world11 and exposure to toxic agents, including lead and other metals, may contribute to the onset or impairment of these pathological processes. Population studies about the cardiovascular effects of lead are focused on the association between its exposure and the development of hypertension. On the other hand, researches in experimental animals seek to elucidate, in addition to the hypertensive effect, the mechanisms involved in the cardiovascular alterations promoted by this metal.9
It should be emphasized that when analyzing the effects of lead on the vascular system, the type of vascular bed studied (resistance or conductance) should be considered. In addition, other factors such as: dose, time and route of exposure to the metal must be observed, since all of them influence in a different way the results provoked by this cation in the biological organisms.
In this review will be emphasized the toxic effects of lead, on conductance vessels, especially the aorta artery. We will present studies in humans and experimental animals that demonstrated the relationship between acute and chronic exposure to this metal with vascular diseases.
– Acute and chronic vascular effects of lead in humans and experimental animals
Furchgott & Vanhoutte12 described for the first time the role of the endothelium in the regulation of vascular tone. From this study blood vessels are no longer seen merely as conductors of humors in the circulatory system. It is now known that the endothelium is able to release metabolically active substances, which modulate important functions in the body such as vasomotor tone control, vascular caliber, blood flow and also participates in the control of inflammatory and immune responses.12,13 In fact, the functions of the vascular system imply complex interactions between endothelium, vascular smooth muscle, immune system, nervous system. Moreover, these bonds are present in the chemical and metabolic processes of individual organs.14-17
In this sense, several studies have pointed out that the toxicity of heavy metals (lead, mercury, cadmium, copper, arsenic) targets the vascular system promoting damage ranging from hemorrhagic lesions to other pathological processes including edema, atherosclerosis and arterial hypertension. In addition, the vascular effects of these elements can act on specific organs impairing the metabolic processes and cellular remodeling.18-22
Several mechanisms have been proposed to explain the genesis of arterial hypertension caused by lead mainly involving interactions of this metal with endogenous regulatory processes present in endothelial cells and vascular smooth muscle. These factors include: inhibition of Na+/K+ -ATPase; Increased renin-angiotensin system activity,23-27 endothelial dysfunction,27-29 and vascular smooth muscle.30-36
In recent decades, lead-related research has focused on its direct and / or indirect effects on vascular structure, both in studies in humans and in experimental animals. However, there is still no consensus among the authors regarding the pathways of action of lead in the biological organism.
Barbosa Jr. et al,4 studied 62 volunteers living in the city of Bauru (SP- Brazil) who were exposed to lead in the environment. These authors demonstrated a negative correlation between plasma nitrite and lead present in the plasma and blood of these volunteers, suggesting that this metal promotes an inhibitory effect on the formation of nitric oxide (NO). This finding is supported by other studies whose results have shown that measurements of plasma nitrite concentration reflect the activity of the enzyme nitric oxide synthase (NOS).37-39 Den Hond, et al,40 using data from the Third National Health and Nutrition Examination Survey (NHANES III) found a positive correlation between lead concentration and increased blood pressure in black women and men.
In a series of studies conducted in our laboratory we have demonstrated that acute and chronic exposure to lead increases the blood pressure of rats associated with vascular dysfunction.41,42
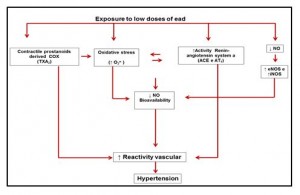
These studies revealed differentiated responses depending on the time and route of exposure to lead. Silveira et al.,43 verified the acute effects of lead in the caudal bed of rats acutely exposed to high concentrations of lead (100 μΜ). The findings of this study reveal an increase in the reactivity of the caudal arteries to phenylephrine (α 1 -adrenergic agonist). This response was dependent on the endothelium and the functional changes observed in these arteries suggest the local involvement of the following pathways: nitric oxide; Cyclooxygenase and reactive oxygen species. The main findings of this study can be verified in figure (1) below.
Figure 1: Representing the endothelial pathways involved in the effect of lead on the aorta artery of rats exposed to low doses of lead acetate (Silveira et al., 2010).
In other studies performed in our laboratory26,44 the animals were exposed for 7 days at low doses (via intramuscular injections) of lead. After this period, a blood concentration of lead of 9.98 ± 1.70 μg/dL was observed in exposed animals. The functional and biochemical analyzes of these studies showed a reduction in the contractile response to phenylephrine and greater bioavailability of NO in aortic rings.44 They also observed increased free radical production, potassium channel activation, and increased Na+/K+ -ATPase activity.26 The authors of these studies suggest that increased NO and activation of potassium channels and sodium pump may reduce vascular tone in the early stages of exposure to lead by counteracting the vasoconstricting actions of free radicals.
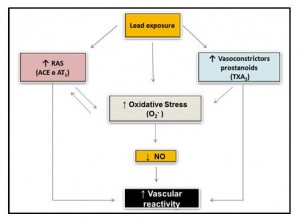
On the other hand, Silveira et al,27 studied the effects of lead 30 days after daily exposure (intramuscular injections) at low doses of this metal. After this period the exposed rats had a blood concentration of 12 ± 1.34 μg/dL. In this study, there was an increase in systolic blood pressure associated to functional and biochemical alterations analyzed in aortic rings of experimental animals. These authors propose that the increase in vascular reactivity can be attributed to the reduction in NO bioavailability caused by increased production of reactive oxygen species via NAD(P)H oxidase and also to the lower negative endothelial modulation, involving greater participation of the superoxide anion, of contractile prostanoids derived from COX, especially TXA2 and participation of the renin-angiotensin system in this response. The functional results of this study were confirmed by some biochemical findings that showed an increase in the protein expression of the eNOS and iNOS isoforms and the AT1 receptor. The main results of this study are summarized in figure (2) below.
Figure 2: Representation of the endothelial pathways involved in the effect of lead on the rat aorta artery (Silveira et al., 2014).
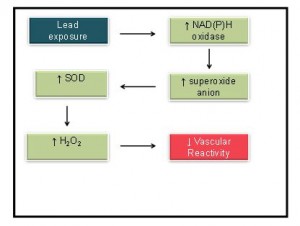
Finally, Nunes et al,45 investigated the effects of lead on the vascular reactivity of rat aortic arteries after 30 days of daily exposure to 100 ppm of lead in drinking water. The blood concentration of the animals exposed to lead was 8.4 μg/dL ± 1.1 μg/dL. The findings of this research revealed an increase in the production of superoxide anion (O2-) by NAD(P)H oxidase triggering a compensatory increase in the activity of the enzyme superoxide dismutase (SOD). According to the authors, the increase in the activity of this enzyme increased the production of hydrogen peroxide (H2O2), which was responsible for the decrease in vascular reactivity. Figure 3 illustrates the main findings of this study.
Figure 3: Representation of the main endothelial pathways in aorta after rings 30 days exposure to 100 ppm of lead acetate in drinking water (Nunes et al., 2015).
Analyzing the main findings found in the researches of Silveira et al,27,43 Fiorim et al.,26,44 and Nunes et al.,45 we can observe that the deleterious effects of lead in the vascular reactivity of conductance vessels are dependent of time of exposure and also the way of exposure. This difference is clearly demonstrated in the results that showed increase27,44 and reduction of vascular reactivity.26,44, 45
Conclusion
The studies presented in this review showed that lead promotes vascular changes resulting in increased blood pressure. It should be noted that these alterations are present in studies performed in both humans and experimental animals. It is known that cardiovascular diseases represent one of the main causes of death and morbidity in Brazil and in other countries. Therefore, we ratify the need to alert the toxicological
surveillance agencies of the levels of lead exposure considered safe for individuals exposed to occupational and environmental contamination of this metal. In addition, it is important to include lead as a risk factor for the development of various cardiovascular diseases.
References
- Smith AH; Sciortino S; Goeden H; Wright CC. Consideration of background exposure in the management of hazardous waste sites: A New Approach to Rick Assessment. Risk Analysis. 1996;16(5):619-625.
- Barry, PS. A comparison of concentrations of lead in human tissues. Br J Ind Med. 1975;32:119-139.
- Keogh JP; Boyer LV. Lead – Specific Health Hazards and Toxins. In_: Clinical Environmental Health and Toxic Exposures. Sullivan, J.B. and Krieger, G.R. (eds). 2001. Williams & WilKins, Philadelphia, USA.
- Barbosa FJr; Tanus-Santos, JE; Gerlach RF; Parsons, PJ. A Critical Review of Biomarkers Used for Monitoring Human Exposure to Lead: Advantages, Limitations, and Future Needs. Environtal Health Persp. 2005;13:1669-74.
- Wedeen RP. Bone Lead, Hypertension, and Lead Nephropathy. Environtal Health Persp. 1988;78:57-60.
- Beevers DG, Erskine E, Robertson M, Beattie AD, Campbell BC, Goldberg A, Moore MR, Hawthorne VM. Blood-lead and hypertension. 1976;2:1-3.
- Harlan, WR; Landis, JR; Schmouder, RL; Goldstein, NG; Harlan, LC. Blood lead and blood pressure. Relationship in the adolescent and adult US population. JAMA. 1985;253:530-534.
- Schwartz J.The Relationship Between Blood Lead and Blood Pressure in the NHANES II Survey. Environtal Health Persp. 1988;78:15-22.
- Navas-Acien A, Guallar E, Silbergeld EK, Rothenberg SJ. Lead exposure and cardiovascular disease a systematic review. Environ Health Perspect. 2007;115:472-482.
- Menke A; Muntner P; Batuman V; Silbergeld EK; Guallar E. Blood lead below 0.48 micromol/L (10 microg/dL) and mortality among US adults. 2006;114(13):1388-94.
- World Health Organization (WHO) (2011) Cardiovascular Diseases. Available: http://www.who.int/cardiovasculardiseases/en/.
- Furchgott, RF; Zawadzki, JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcoline. Nature. 1980;288:373-376.
- Rubanyi GM. The role of endothelium in cardiovascular homeostasis and diseases. J Cardiovasc Pharmacol. 1993;22 Suppl 4: S1-14.
- Galley HF, Webster NR. Physiology of the endothelium. Br J Anaesth. 2004;93(1):105-13.
- Gibbins IL, Jobling P, Morris JL. Functional organization of peripheral vasomotor pathways. Acta PhysiolScand. 2003;177(3):237-45.
- Hill MD, Silver FL. Epidemiologic predictors of 30-daysurvival in cerebellar hemorrhage. J Stroke Cerebrovasc Dis. 2001;10(3):118-21.
- Villar IC, Francis S, Webb A, Hobbs AJ, Ahluwalia A. Novel aspects of endothelium-dependent regulation of vascular tone. Kidney Int. 2006;70(5):840-53.
- Navas-Acien A, Sharrett AR, Silbergeld EK, Schwartz BS, Nachman KE, Burke TA, Guallar E. Arsenic exposure and cardiovascular disease: a systematic review of the epidemiologic evidence. Am J Epidemiol. 2005;162(11):1037-49.
- Prozialeck WC, Edwards JR, Woods JM. The vascular endothelium as a target of cadmium toxicity. Life Sci. 2006;79(16):1493-506.
- Liu F; Inageda K; Nishitai G; Matsuoka M. Cadmium Induces the Expression of Grp78, an endoplasmic reticulum molecular chaperone, in LLC-PK1 renal epithelial cells. Environ Health Perspect. 2006;114(6):859-64.
- Vassallo DV; Simões MR; Furieri LB; Fioresi M; Fiorim J; Silveria EA; et al. Toxic effects of mercury, lead and gadoliniumon vascular reactivity. Braz J Med Biol Res. 2011;44:939-946.
- Liu K; Gu P; Chen W; Shi J; Xia L. Effect of Pregnancy on the Levels of Blood Cadmium and Lead: analysis of 2006-2011 Nanjing Maternity and Child Health Care Hospital Survey Data. Iranian J Publ Health. 2013;42(7):691-699.
- Weiler E, Khalil-Manesh F; Gonick H. Effects of Lead and Natriuretic Hormone on Kinetics of Sodium-Potassium Activated AdenosineTriphosphatase: Possible Relevance to Hypertension. Environ Health Perspect. 1988;78:113-115.
- Sharifi, MA; Darabi, R; Akbarloo, N; Larijani, B; Khoshbaten, A. Investigation of circulatory and tissue ACE activity during development of lead-induced hypertension. Toxicol Lett. 2004;153:233-238.
- Simões MR, Ribeiro Júnior RF, Vescovi MV, de Jesus HC, Padilha AS, Stefanon I, et al. M. Acute lead exposure increases arterial pressure: role of the Renin-Angiotensin system. PloSOne. 2011;6(4):e18730.
- Fiorim J, Ribeiro Júnior RF, Azevedo BF, Simões BMR, Padilha AS, Stefanon I, et al. DV. Activation of K+ channels and Na+/K+ATPase prevents aortic endothelial dysfunction in 7-day lead-treated rats.Toxicol Appl Pharm. 2012;262:22-31.
- Silveira EA, Siman FD, de Oliveira Faria T, Vescovi MV, Furieri LB, Lizardo JH, et al. Low-dose chronic lead exposure increases systolic arterial pressure and vascular reactivity of rat aortas. Free Radic Biol Med. 2014; 67: 366-76.
- Vaziri, ND; Liang, K; Ding, Y. Increased nitric oxide inactivation by reactive oxygen species in lead-induced hypertension. Kidney Int. 1999;56:1492-98.
- Vaziri, ND. Mechanismsof lead-inducedhypertensionand cardiovascular disease.Am J Physiol Heart Circ Physiol.2008; 295:H454–H465.
- Webb RC, Winquist RJ, Victery W, Vander AJ. In vivo and in vitro effects of lead on vascular reactivity in rats. Am J Physiol.1981;241: H211-H216.
- Tomera, JF, Harakal C. Mercury and lead-induced contraction of aortic smooth muscle in vitro. Arch Int Pharmacodyn Ther. 1986;283: 295-302.
- Chai S, Webb RC. Effects of lead on vascular reactivity. Environtal Health Persp. 1988;78:85-89.
- Watts SW, Chai S, Webb RC. Lead acetate-inducedcontraction in rabbitmesentericartery: interaction with calcium and protein kinase C. Toxicol. 1995;99(1-2):55-65.
- Fujiwara Y, Kaji T, Yamamoto C, Sakamoto M, Kozuka H. Stimulatory effect of lead on the proliferation of cultured vascular smooth-muscle cells. Toxicol. 1995;98(1-3):105-10.
- Kaji T, Fujiwara Y, Hoshino M, Yamamoto C, Sakamoto M, Kozuka H. Inhibitory effect of lead on the proliferation of cultured vascular endotelial cells. Toxicol. 1995;95(1-3):87-92.
- Marques M, Millás I, Jiménez A, García-Colis E, Rodriguez-Feo JA, Velasco S; et al. Alteration of the soluble guanylate cyclase system in the vascular wall of lead-induced hypertension in rats. J Am Soc Nephrol. 2001;12:2594–600
- Kelm M, Preik-Steinhoff H, Preik M, Strauer BE. Serum nitrite sensitively reflects endothelial NO formation in human forearm vasculature: evidence for biochemical assess ment of the endothelial L-arginine–NO pathway. Cardiovasc Res. 1999; 41:765-772.
- Lauer T, Preik M, Rassaf T, Strauer BE, Feelisch ADM, Kelm M. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. 2001;98(22):12814–819.
- Kleinbongard P, Dejam A, Lauer T, Jax T, Kerber S, Gharini P, et al. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic Biol Med. 2006;40(2):295-302.
- DenHond E; Nawrot T; Staessen JA. The relationship between blood pressure and blood lead in NHANES III National Health and Nutritio Examination Survey. J Hum Hypertens. 2002;16:563-568.
- Stöfen D. Environmental lead and the heart. J Mol Cell Cardiol. 1974;6:285-90.
- Dimitrova, M. Changes in the contractile function of the myocardium in chronic saturnism. Arch Mal Prof. 1972;33(7):383-8.
- Silveira, EA; Lizardo, JH; Souza, LP; Stefanon, I; Vassallo, DV. Acute lead-induced vasoconstriction in the vascular beds of isolated perfused rat tails is endothelium dependent. Braz J Med Biol Res.2010;43(5):492-9.
- Fiorim J, Ribeiro Júnior RF, Silveira EA, Padilha AS, Vescovi MV; de Jesus HC, et al. Low-level lead exposure increases systolic arterial pressure and endothelium derived vasodilator factors in rat aortas. PLoSOne. 2011;6:e17117.
- Nunes ZK, Nunes DN, Silveira EA, Pereira CAC, Broseghini Filho GB, Vassallo VD, et al. Chronic lead exposure decreases the vascular reactivity of rat aortas: The Role of Hydrogen Peroxide. PLoSOne. 2015;10(3):e0120965.
Authors
Edna Aparecida Silveira1; Maylla Ronacher Simões2, Jonaína Fiorim3; Mirian Fioresi4
1,3Doctors in Physiological Sciences at PPGCF-UFES; Physiotherapists in University Hospital Cassiano Antonio Moraes, HUCAM.
2Doctor in Physiological Sciences by PPGCF-UFES; Post-doctoral stage, PPGCF-UFES.
4Doctor in Physiological Sciences by the Post-Graduation Program in Physiological Sciences of the Federal University of Espírito Santo, PPGCF-UFES; Professor, Department of Nursing, UFES, Brazil.